Sensitization & Irritation Testing
Accurate assessment of material safety requires a detailed understanding of how materials interact with biological tissues and immune systems upon contact. Sensitization & Irritation Testing evaluates whether materials trigger allergic responses, immune activation, or localized inflammatory effects when exposed to skin, mucosal surfaces, or tissues during intended use.
At Materials Metric, we conduct sensitization and irritation studies aligned with ISO 10993-10, ISO 10993-23, and FDA guidance, providing clear, defensible data to support biocompatibility evaluations and regulatory submissions. Sensitization testing focuses on delayed hypersensitivity and immune-mediated responses, while irritation testing assesses localized tissue reactions such as erythema, edema, and structural disruption. Together, these studies form a critical component of biological risk assessment for medical devices, biomaterials, and patient-contacting products.
Uses of Sensitization & Irritation Testing
• Screening for allergic or inflammatory potential
• Evaluating biomaterial surface chemistry, coatings, or residues
• Comparing new or modified formulations
• Ensuring safety for skin-contacting or mucosal-contacting devices
• Supporting ISO 10993 panels for FDA submissions

Applications
• Wearable devices, adhesives, and dressings
• Dental polymers and restoratives
• Wound-care materials
• Elastomers, hydrogels, and coatings
• Consumer products and cosmetic-contacting materials
Sample Analysis Process
1. Study Planning
- Determine if irritation, sensitization, or both are required
- Select in-vitro irritation models or validated sensitization assays
2. Sample Preparation
- Extract preparation per ISO 10993-12
- Application to model systems or in-vitro irritation membranes
3. Exposure & Measurement
- Monitoring of inflammatory or immune response markers
- Quantitative irritation scoring
- Sensitization pathway activation (if applicable)
4. Reporting
- Irritation indices
- Sensitization activation results
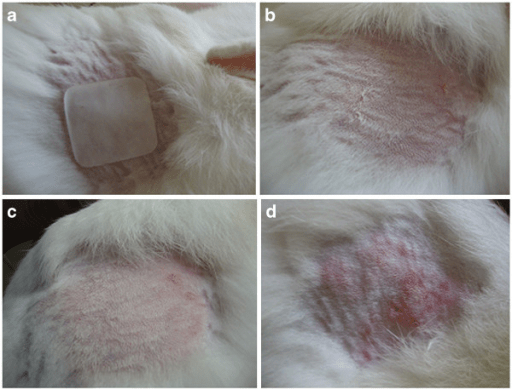
- Photographs/annotated images
- ISO 10993-10 classification and regulatory interpretation
Why Choose Materials Metric
Materials Metric delivers high-fidelity sensitization and irritation testing supported by deep expertise in inflammatory pathways, biomaterial responses, and regulatory biocompatibility requirements.
We provide:
- ISO 10993-10–aligned protocols designed for global regulatory acceptance
- ISO 9001:2015 accredited processes ensuring reproducible, traceable, and audit-ready results
- Modern in-vitro irritation and immune-response assays offering faster, more ethical alternatives to outdated methods
- Expert interpretation from toxicologists and biomaterials scientists who understand the mechanisms driving sensitization or irritation
- Integrated chemical + biological analysis to link adverse responses to specific leachables, residues, or surface features
Materials Metric delivers high-confidence biological safety insights that strengthen FDA submissions and reduce regulatory risk.
Related services
