Immunological & Cell-Based Assays
Immunological and cell-based assays evaluate cellular behavior, immune response, cytokine signaling, biomarker expression, and functional activity in response to biomaterials, therapeutics, devices, coatings, and engineered systems. These assays provide essential insights into biocompatibility, toxicity, inflammation, immunomodulation, therapeutic performance, and biological function, supporting both early-stage R&D and regulatory submissions.
These methods encompass immune cell activation profiles, cytokine release, macrophage polarization, cytotoxicity, proliferation, migration, phagocytosis, functional cell assays, and cellular interaction testing, providing a comprehensive biological readout of how materials or therapeutic candidates perform in a biological environment.
Materials Metric offers a full suite of immunological and cell-based assays including ELISA, multiplex cytokine assays, functional cellular assays, flow cytometry, and targeted immunoassays constructed to support developers in biomaterials, medical devices, pharmaceuticals, regenerative medicine, tissue engineering, and antimicrobial technologies.
Immunological & Cell-Based Assays Can Achieve
1. Immune Response & Inflammation Profiling
Cytokine and chemokine quantification (IL-1β, IL-6, TNF-α, IL-10, etc.)
Macrophage activation and M1/M2 polarization assays
Lymphocyte activation, proliferation, and immune compatibility
Assessment of foreign body response
2. Cytotoxicity & Cell Viability Analysis
Live/dead assays, metabolic activity (MTT/Alamar Blue), ATP quantification
Evaluation of leachables, degradation byproducts, or extracts
Time-dependent cytotoxicity studies
3. Functional Cell Assays
Migration and wound-healing assays
Phagocytosis assays
Barrier integrity and TEER measurements
Contractility assays (cardiac, muscle, engineered tissues)
4. Cell–Material Interaction Studies
Adhesion, spreading, and proliferation
Cell infiltration into scaffolds or hydrogels
ECM (collagen, elastin, GAG) production and remodeling
Protein adsorption and surface–cell interaction analysis
5. Mechanistic Biomarker & Gene Expression Analysis
qPCR or immunoassay-based pathway analysis
Stress-marker profiling and apoptosis assays
Mechanistic insight into toxicity, repair, regeneration, or immune activation


Applications Across Biomedical & Materials R&D
Biomaterials & Medical Devices
Evaluating host response to implants, coatings, nanoparticles, scaffolds
Screening materials for inflammatory or immunomodulatory properties
Pharmaceutical & Therapeutic Development
Assessing drug mechanism of action
Potency and efficacy testing using physiologically relevant cell models
Tissue Engineering & Regenerative Medicine
Monitoring differentiation, ECM deposition, and tissue remodeling
Investigating immune–biomaterial interactions
Antimicrobial & Biofilm Research
Immune response to biofilm-associated infections
Interaction of antimicrobial coatings with immune cells
Immunological & Cell-Based Assay Workflow
1. Study Design & Experimental Planning
Selection of cell types (immune cells, primary cells, stem cells, functional tissues)
Define biomarkers, functional endpoints, and biological mechanisms of interest
2. Sample Preparation & Culture Setup
Sterilization and conditioning of materials or device extracts
2D, 3D, co-culture, or engineered tissue systems
3. Assay Execution
Cytotoxicity, viability, and proliferation assays
Cytokine profiling (ELISA, multiplex assays)
Functional assays (migration, phagocytosis, TEER, contractility)
Gene expression and protein analysis
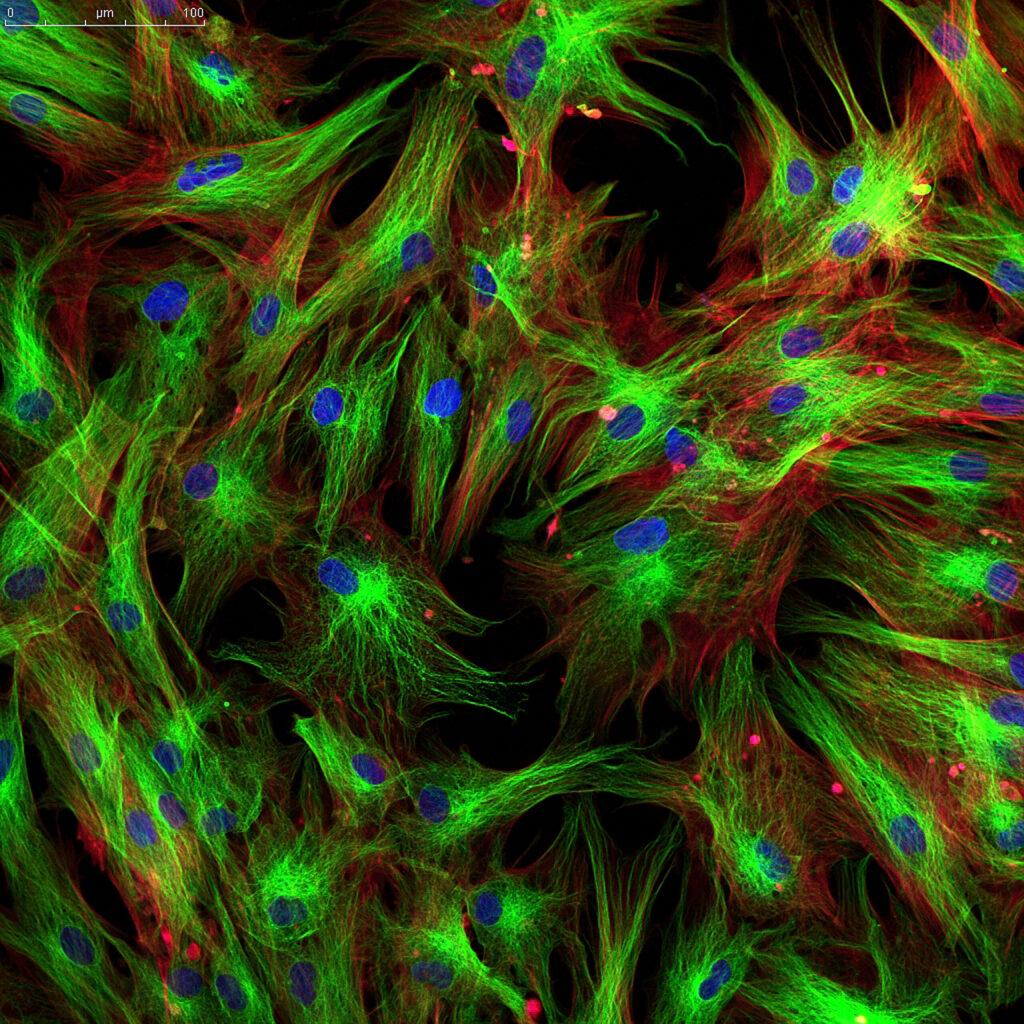
4. Imaging & Structural Assessment
Confocal, fluorescence, or brightfield imaging
SEM for surface–cell interactions
Histology and immunostaining
5. Data Quantification & Interpretation
Statistical analysis and biomarker interpretation
Mechanistic understanding of cell or immune behavior
Comparative evaluation across materials, formulations, or treatment groups
6. Reporting & Recommendations
Comprehensive, publication-ready data package
Insight-driven conclusions for R&D progression
Optional regulatory-supportive documentation
Why Choose Materials Metric
Materials Metric combines deep biological expertise, advanced imaging platforms, and analytical precision to deliver high-quality immunological and functional cell-based data.
We offer:
ISO 9001:2015–aligned workflows for reproducibility and scientific rigor
Expertise across immunology, cellular biology, biomaterials, analytical chemistry, and microscopy
Integrated testing capabilities linking biological response with material, chemical, and mechanical properties
Custom assay design tailored to your device, therapeutic, or biomaterial system
Detailed reporting suitable for regulatory submissions, R&D strategy, and scientific publications
Our assays provide a clear, mechanistic understanding of how materials and therapeutics interact with biological systems, empowering confident innovation and development.
