Biofilm Formation & Resistance Studies
Biofilm Formation & Resistance Studies examine how microorganisms adhere to material surfaces, proliferate, and develop structured, highly resistant microbial communities under realistic conditions. Biofilms significantly increase tolerance to antibiotics, disinfectants, and environmental stress, making them a critical challenge for medical devices, implants, wound-care systems, water-contact materials, and antimicrobial coatings.
At Materials Metric, we design clinically and application-relevant biofilm models to evaluate surface susceptibility, antimicrobial durability, and resistance mechanisms. By integrating controlled biofilm growth assays, quantitative viability measurements, and structural characterization, we generate actionable insight into how materials perform against one of the most persistent biological threats in real-world use.
Methods & Models Used in Biofilm Testing
Materials Metric performs both standardized and custom-designed biofilm models tailored to your product’s clinical or functional use.
Common Biofilm Assay Types
Static biofilm models
Surface attachment & growth on coupons, films, implants, coatingsDynamic flow systems
Flow cells simulating real physiological or environmental flowCDC Biofilm Reactor®
Industry-standard system for reproducible biofilm formationMicrotiter plate assays
Crystal violet staining, metabolic assays, viability stainingConfocal microscopy-based 3D biofilm imaging
Structural analysis and viability mappingCustom microbial challenge models
For novel materials, wound dressings, or antimicrobial surfaces
Microorganisms commonly tested
Staphylococcus aureus
Staphylococcus epidermidis
Pseudomonas aeruginosa
E. coli
Candida albicans
Mixed-species biofilms
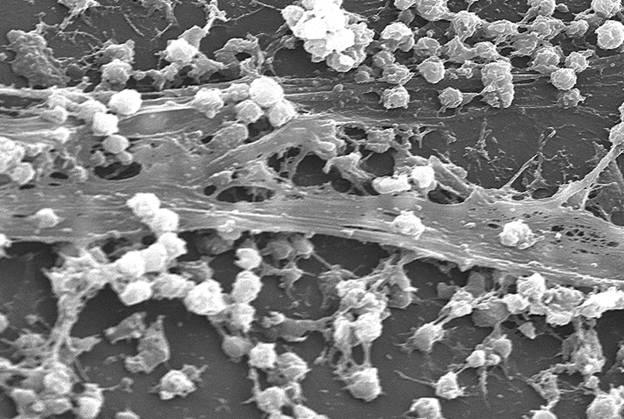
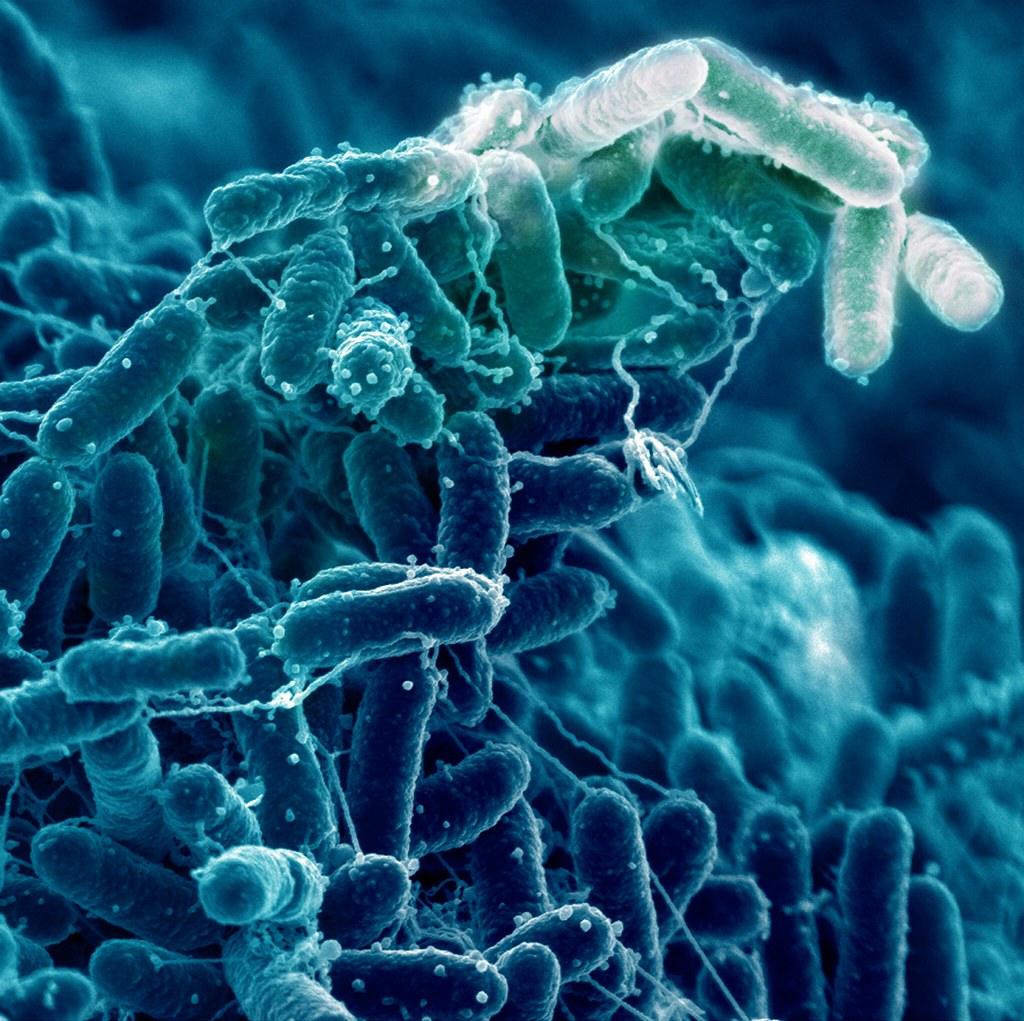
Applications of Biofilm Formation & Resistance Studies
Medical Devices & Implants
Catheters, orthopedic implants, dental materials, wound dressings, meshes, and stents.
Biomaterials & Coatings
Hydrogels, antimicrobial coatings, nanostructured surfaces, and bioactive films.
Water & Environmental Systems
Filters, membranes, piping components, HVAC surfaces, and disinfection technologies.
Pharmaceutical & Topical Formulations
Antibiofilm gels, ointments, sprays, and drug-eluting coatings.
Industrial & Consumer Products
Surface treatments, antimicrobial plastics, and packaging materials.
Sample Analysis Process
1. Study Design & Parameter Selection
Selection of microbial species
Determination of environmental or clinical conditions
Flow/static model selection
2. Surface Preparation & Conditioning
Sterilization, hydration, or coating preparation
Application of antimicrobial or protective treatments
3. Biofilm Growth & Challenge Exposure
Controlled incubation under static or dynamic conditions
Biofilm establishment over hours to weeks
4. Quantitative & Qualitative Analysis
CFU enumeration
Crystal violet staining
Biomass quantification
Viability staining (Live/Dead)
SEM and CLSM imaging for ultrastructure visualization
5. Resistance & Removal Evaluation
Exposure to antimicrobials or cleaning agents
Measurement of remaining viability and biofilm structure
Evaluation of detachment and regrowth behavior
6. Data Interpretation & Reporting
Full dataset with biofilm quantification
Imaging-based evidence
Performance comparison against controls
Recommendations for optimization
Why Choose Materials Metric
Materials Metric provides rigorous, realistic biofilm models that match clinical and industrial conditions.
We offer:
• Controlled flow-cell and static biofilm systems
• ISO 9001:2015 accredited processes
• Expertise in antimicrobial materials and biological interactions
• Integrated imaging (SEM, CLSM, AFM) for structural insights
• Mechanistic interpretation for product optimization
Materials Metric helps you understand how your materials perform against the toughest microbial challenges.
Related services
