Antimicrobial Efficacy Testing
Accurately evaluating antimicrobial performance is critical for materials and products designed to resist microbial contamination, infection, or biofouling. Antimicrobial Efficacy Testing assesses whether materials, coatings, formulations, or devices can inhibit microbial growth, reduce viable pathogen levels, or prevent surface colonization under defined and reproducible conditions.
At Materials Metric, we apply standardized and custom antimicrobial assays, including log-reduction measurements, time-kill studies, zone-of-inhibition testing, and USP, ISO, and ASTM methods to generate quantitative, defensible performance data. These studies support product development, mechanism optimization, and regulatory submissions across medical devices, pharmaceuticals, wound-care systems, consumer products, and antimicrobial surface technologies.
Antimicrobial studies quantify:
Log reduction of viable microbes
Inhibition of growth zones or bacterial spreading
Bactericidal vs. bacteriostatic activity
Time-kill kinetics and rate of microbial reduction
Residual antimicrobial activity over time
Effectiveness under wet, dry, or physiological conditions
Common Test Standards & Methods
Materials Metric provides antimicrobial testing aligned with:
USP 51 (Antimicrobial preservative effectiveness)
ASTM E2315 (Time-kill studies)
ASTM E2149 (Dynamic contact antimicrobial test)
ISO 22196 / JIS Z 2801 (Antimicrobial surface activity)
ASTM E1428 (Challenge tests for biomaterials)
Custom microbial challenge models for novel materials and coatings
We test both Gram-positive and Gram-negative bacteria, fungi, and clinically relevant organisms.

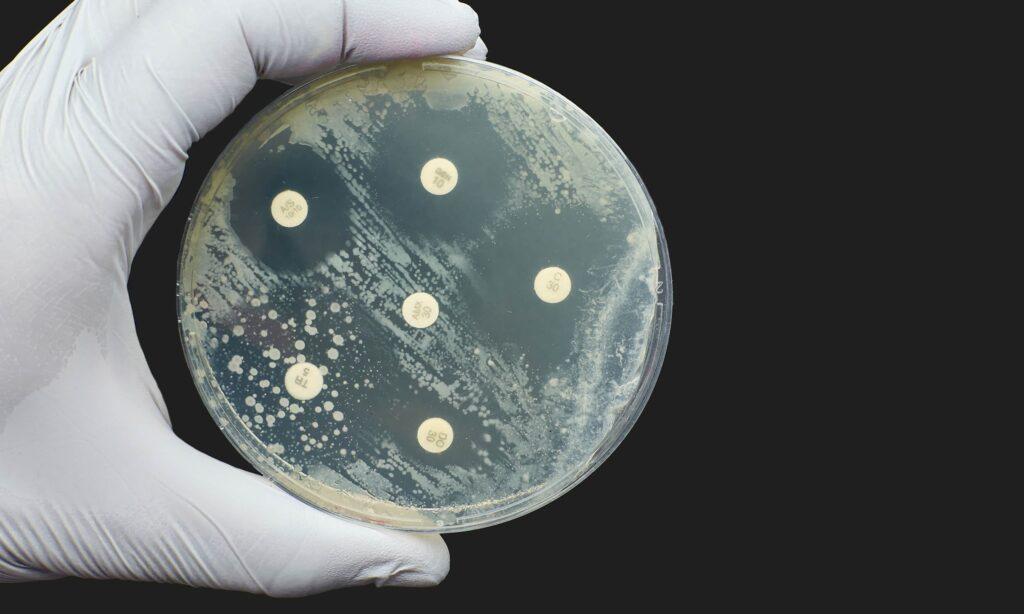
Applications
Medical devices & implants
Catheters, wound dressings, sutures, orthopedic coatings, dental materialsBiomaterials & surface coatings
Antibacterial films, nanostructured surfaces, antimicrobial hydrogelsPharmaceuticals & topical formulations
Creams, gels, ointments, drug-loaded dressingsConsumer products & packaging
Antimicrobial plastics, textiles, personal care productsEnvironmental and industrial systems
Water treatment materials, HVAC coatings, filtration devices
Antimicrobial Efficacy Testing Process
1. Test Planning & Organism Selection
Identify target pathogens (e.g., S. aureus, E. coli, P. aeruginosa, C. albicans)
Define relevant standards, exposure conditions, and performance endpoints
2. Sample Preparation & Conditioning
Surface sterilization when required
Hydration, incubation, or environmental preconditioning
3. Microbial Challenge & Incubation
Apply microbes directly to sample surfaces
Expose samples under controlled temperature, humidity, and growth conditions
4. Quantification of Microbial Reduction
CFU enumeration
Log-reduction calculations
Viability staining for visual confirmation
Time-kill curves
5. Reporting & Interpretation
Full performance summary
Compliance with USP, ISO, ASTM guidelines
Recommendations to improve antimicrobial performance

Why Choose Materials Metric for Antimicrobial Efficacy Testing
Materials Metric provides highly controlled, reproducible antimicrobial testing backed by microbiology, materials science, and regulatory expertise.
We offer:
• ISO 9001:2015 accredited quality systems
• Validated antimicrobial assays including USP, ISO, and ASTM methods
• Quantitative log-reduction analysis with high sensitivity
• Expertise in coatings, hydrogels, polymers, and biomedical materials
• Integrated microscopy and biofilm analysis for deeper mechanistic insight
Materials Metric delivers accurate, regulator-ready antimicrobial performance data you can trust for R&D or submission packages.
