Rapid Diagnostic & Screening Assays
Rapid Diagnostic & Screening Assays are fast, sensitive, and high-throughput analytical methods designed to quickly detect biological markers, pathogens, contaminants, molecular targets, functional activity, or material-induced effects. These assays enable rapid decision-making during early R&D, quality control, safety testing, and preclinical evaluation, without the long turnaround times associated with traditional analytical workflows. Using molecular, biochemical, immunological, and cell-based platforms, these assays provide quick, reliable, and actionable insights, supporting product development across biomaterials, medical devices, pharmaceuticals, antimicrobial technologies, cosmetics, tissue engineering, and diagnostics.
Materials Metric develops and executes rapid diagnostic and screening assays tailored to the unique needs of each project, whether identifying contamination, screening candidate materials, verifying device safety, or quantifying biological response in accelerated timelines.
Rapid Diagnostic & Screening Assays Can Achieve
1. Fast Detection of Contaminants & Microbes
Rapid bacterial, fungal, or microbial detection
Endotoxin and pyrogen screening
Early identification of contamination in materials, media, or devices
2. Biomarker & Molecular Signal Screening
Rapid quantification of proteins, cytokines, and signaling molecules
qPCR-based screening for DNA/RNA targets, mutations, or microbial species
Multiplex immunoassay detection of multiple biomarkers simultaneously
3. High-Throughput Material Screening
Cytotoxicity, viability, and metabolic assays for early selection
Rapid compatibility assessment of biomaterials and coatings
Candidate ranking before deep-dive biological evaluations
4. Functional & Biochemical Screening
Enzyme activity and metabolic response
Barrier integrity (TEER) and permeability screens
Wound-healing, migration, and basic antimicrobial efficacy assays
5. Extractables, Leachables & Degradation Screening
Fast assessment of harmful chemical leachables
Detection of early degradation products
Impurity and stability screening during formulation development
6. Device Integrity & Surface Interaction Testing
Rapid surface reactivity assays
Early-stage biological interaction screening before comprehensive testing
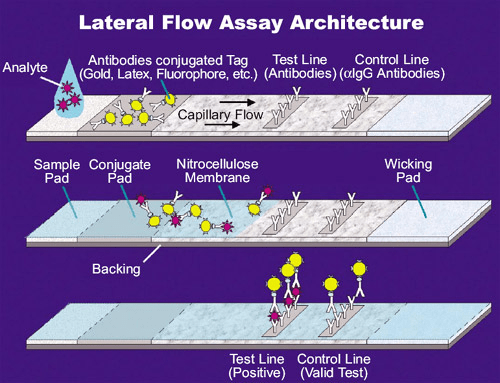
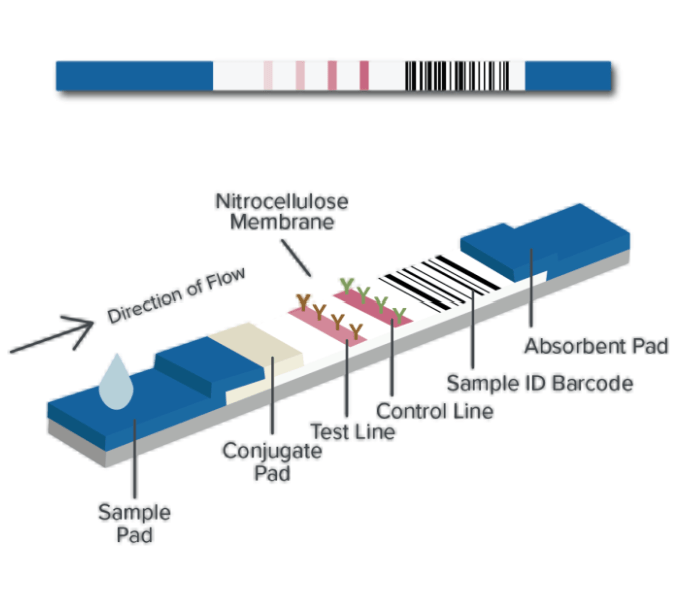
Applications
Pharmaceutical & Therapeutics Development
Early candidate screening
Rapid cytotoxicity, potency, and efficacy evaluations
Biomarker response profiling for mechanism-of-action insights
Medical Devices & Biomaterials
Screening of surface coatings, polymers, hydrogels, ceramics, and implantable materials
Early-stage compatibility testing for device extracts, coatings, and degradation products
Accelerated evaluation for biomaterial QC and lot-release testing
Antimicrobial & Biofilm Research
Fast inhibition assays for antimicrobial materials and coatings
Rapid screening for biofilm suppression or microbial resistance trends
Cosmetics, Dermatology & Personal Care
Rapid irritation, sensitivity, and safety screens
Quick evaluation of topical, dermal, and transdermal formulations
Quality Control & Manufacturing
Contamination detection for raw materials, intermediates, and finished products
Batch-to-batch consistency monitoring for chemical and biological performance
Medical Diagnostics
Rapid biomarker and molecular signal detection
Screening assays for pathogen identification or diagnostic reagent validation
Early-stage diagnostic assay prototyping
Environmental & Industrial Monitoring
Detection of microbial contamination in water systems, cleanrooms, air-handling units, and industrial processes
Screening for chemical impurities, residues, or biohazards
Environmental monitoring assays for regulatory compliance
Consumer Products Testing
Rapid safety and irritation evaluations for hygiene, skincare, and household products
Screening antimicrobial efficacy in treated fabrics, coatings, and packaged goods
Quick detection of contaminants that impact consumer product quality
Rapid Diagnostic & Screening Assay Workflow
1. Study Objective & Assay Selection
Define the target molecule, organism, or functional endpoint
Choose rapid platforms (qPCR, ELISA, ATP assays, MTT, colorimetric detection, rapid microbial screens)
2. Sample Preparation & Setup
Extract preparation, sterilization, and conditioning
Setup of molecular, biochemical, or cell-based systems
3. Rapid Assay Execution
Viability & cytotoxicity (MTT, Alamar Blue, ATP)
Multiplex cytokine or protein detection
Fast microbial detection
Quick-response molecular assays
4. Data Acquisition & Analysis
High-throughput quantitative output
Comparative ranking across materials or formulations
Standard curve analysis and threshold determination
5. Reporting & Recommendations
Clear, concise summaries of pass/fail outcomes
Identification of promising candidates
Recommendations for confirmatory testing

Why Choose Materials Metric
Materials Metric offers scientifically rigorous, ISO 9001:2015–aligned rapid testing, backed by multidisciplinary expertise across molecular biology, immunology, materials science, microbiology, and analytical chemistry.
We provide:
Fast, reliable turnaround times without compromising accuracy
Expertise in both biological and material-focused rapid screening
High-throughput capabilities for ranking candidates and accelerating R&D
Integrated workflows connecting rapid screens to in-depth analytical and biological studies
Publication-quality, regulatory-supportive data packages
A collaborative approach focused on reducing development risk and accelerating innovation
Our rapid assays empower teams to make confident decisions early, streamline development, and focus resources on the most promising materials or therapeutic candidates.
Related services
