Decellularization & Scaffold Characterization
Decellularization removes cellular components from tissues while preserving the structural, biochemical, and mechanical integrity of the extracellular matrix (ECM). The resulting acellular scaffold provides a biologically compatible framework for tissue engineering, regenerative medicine, implant development, and in-vitro modeling.
Scaffold characterization evaluates the structural, mechanical, biochemical, and functional properties of these decellularized matrices, ensuring they are safe, reproducible, and suitable for downstream applications. This includes assessing ECM composition, porosity, fiber alignment, mechanical strength, biocompatibility, degradation behavior, and microstructure.
Materials Metric provides comprehensive decellularization and scaffold analysis services designed to support academic research, biotechnology companies, medical device developers, and regenerative medicine innovators.
Decellularization & Scaffold Characterization Can Achieve
1. Effective Removal of Cellular Components
Elimination of DNA, lipids, and cellular debris
Preservation of ECM architecture, collagen networks, and bioactive components
Reduction of immunogenicity for improved biocompatibility
2. Structural & Microarchitectural Evaluation
SEM, TEM, CLSM, and imaging to assess porosity, fiber orientation, and surface morphology
Quantitative analysis of pore size, scaffold uniformity, and ECM integrity
3. Mechanical & Functional Characterization
Tensile, compression, shear, and viscoelastic evaluations
Elastic modulus, stiffness, and load-bearing capacity
Degradation kinetics and swelling behavior
4. Biochemical Composition Analysis
Collagen, elastin, and glycosaminoglycan (GAG) quantification
Residual DNA analysis
Protein and ECM marker profiling
5. Biocompatibility & Cell–Scaffold Interaction Assessment
Cytotoxicity and viability assays
Cell adhesion, proliferation, and infiltration studies
Histology and immunostaining for ECM remodeling

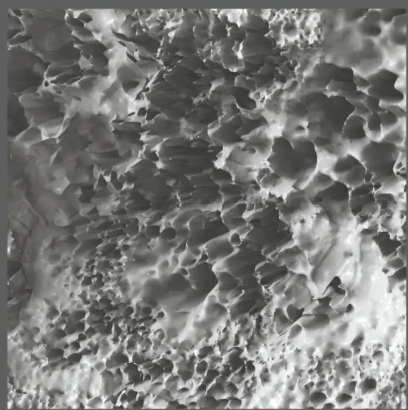
Applications We Support
Regenerative Medicine
Skin, bone, cartilage, cardiac, vascular, nerve, and organ scaffolds
ECM-based graft development
Medical Device Development
Bioactive implants and hybrid synthetic–biological devices
Scaffold-integrated coatings and surface treatments
In-Vitro Models & Drug Screening
Organotypic models for therapeutic testing
Disease modeling using ECM-derived structures
Biomaterial Optimization
Comparing decellularization methods
Establishing ideal scaffold architecture for target tissues
Decellularization & Scaffold Characterization Workflow
1. Tissue Assessment & Decellularization Strategy
Selection of enzymatic, detergent-based, perfusion, mechanical, or combined protocols
Optimization of process conditions for ECM preservation
2. Decellularization Execution
Controlled removal of cellular components
Multiple washing cycles to eliminate chemical residues
3. Structural & Mechanical Characterization
Microscopy (SEM/CLSM/TEM) for surface and internal architecture
Mechanical testing for modulus, strength, and elasticity
Porosity and pore size distribution analysis
4. Biochemical & Molecular Analysis
Residual DNA quantification
GAG, collagen, elastin, and ECM composition measurement
5. Biological Validation
Cell attachment and proliferation assays
Histology, immunostaining, and live/dead imaging
6. Reporting & Recommendations
Comprehensive annotated report with visual data
Optimization guidance for scaffold design
Recommendations for downstream experiments
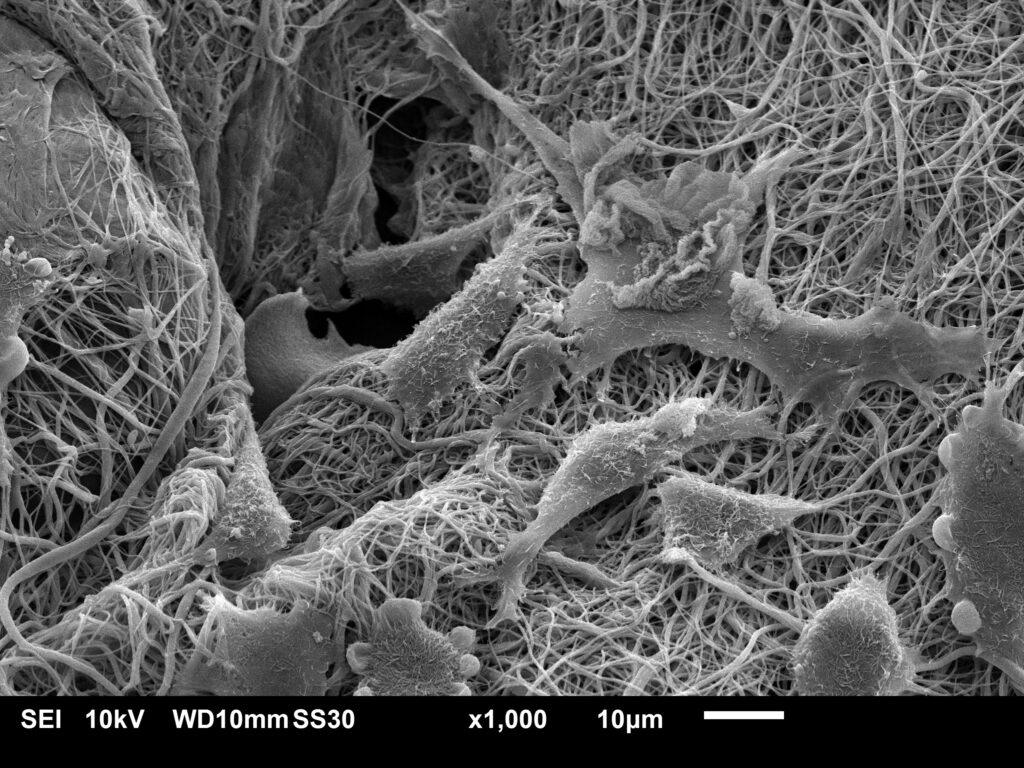
Why Choose Materials Metric
Materials Metric combines advanced analytical infrastructure with deep expertise in biomaterials, tissue engineering, and cellular biology. Our ISO 9001:2015–aligned workflows ensure consistency, rigor, and reproducibility.
We offer:
Extensive analytical capabilities (SEM, TEM, AFM, collagen/GAG assays, mechanical testing, histology, biochemical analysis)
Customizable decellularization protocols for diverse tissues
Cross-disciplinary expertise spanning biomaterials, biomechanics, histology, and cellular assays
Integrated biological and materials evaluation, ensuring comprehensive scaffold characterization
Regulatory-aligned documentation suitable for preclinical development and research submissions
Our holistic approach ensures that your scaffolds are biocompatible, structurally stable, and optimized for regenerative applications.
