Pharmacokinetic & Pharmacometric Modeling
Pharmacokinetic (PK) and pharmacometric (PMX) modeling quantify how drugs move through the body-absorption, distribution, metabolism, and elimination. These models predict exposure, optimize dosing, and support preclinical-to-clinical translation. Materials Metric provides non-compartmental analysis (NCA), compartmental modeling, PK/PD modeling, and population PK (PopPK) studies using validated workflows aligned with FDA and EMA expectations.
Our analytical approach integrates experimental data, simulation tools, and statistical modeling to generate clear insights into drug transport, controlled release, degradation kinetics, and material–tissue interactions. These methods support formulation development, delivery system optimization, and model-informed decision-making across biomedical, pharmaceutical, and biomaterials applications.
Core Capabilities
Pharmacokinetic (PK) Modeling
• NCA for early exposure and clearance assessment
• One- and multi-compartment models
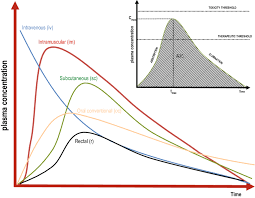
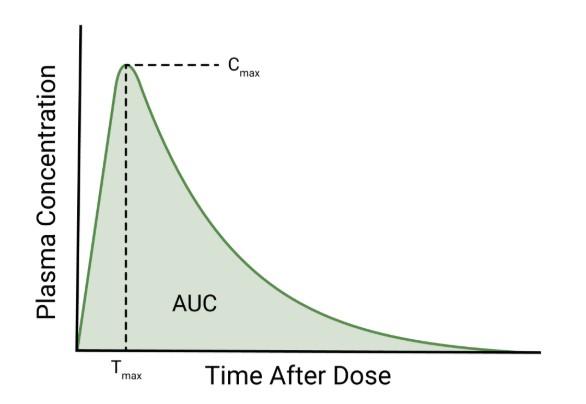
• Time–concentration curve modeling
• AUC, Cmax, Tmax, half-life, and clearance estimation
• PK modeling for implants, hydrogels, coatings, and controlled-release systems
Pharmacometric (PMX) & Population Modeling
• NLME/PopPK modeling to characterize variability
• Exposure–response and PK/PD relationships
• Covariate analysis (age, weight, formulation, disease state)
• Simulation of dosing strategies and regimen optimization
• Scenario testing for preclinical and translational planning
Material-Driven PK/PMX Modeling
• Drug release kinetics from polymers, scaffolds, coatings, and biomaterial surfaces
• Degradation-linked release modeling
• Transport behavior across tissues and biological interfaces
• Integration with histology, imaging, and bio-interface data
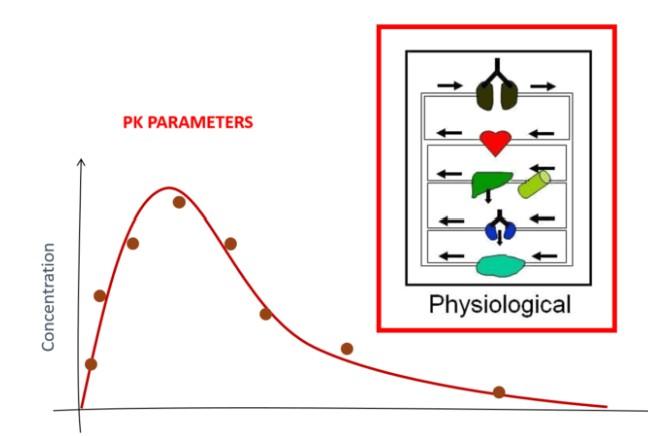
Data Integration & Model Evaluation
• Curve fitting and kinetic parameter estimation
• Bayesian or frequentist estimation workflows
• Simulation of exposure profiles
• Dose-adjustment modeling and sensitivity analysis
• Model diagnostics: goodness-of-fit, residuals, VPCs, and uncertainty analysis
• Data visualization for manuscripts, regulatory filings, and presentations
Types of Studies Supported
• Controlled-release and sustained-delivery platforms
• Polymer, hydrogel, and surface-engineered drug delivery systems
• Local vs systemic exposure prediction
• Implantable depots and transdermal or transmucosal systems
• Regenerative medicine and wound-healing therapeutics
• Bone, cartilage, and soft-tissue delivery models
• Antibiotic, growth factor, and anti-inflammatory release kinetics
Why Choose Materials Metric
Materials Metric offers quantitative modeling with deep biological and statistical insight, backed by ISO 9001:2015 quality controls.
We provide:
• FDA-aligned PK/PD modeling
• PopPK models for inter-individual variability
• Integration with toxicology and efficacy data
• Expert interpretation for regulatory filings
• Scenario simulations that accelerate decision-making
Related services
