Cytotoxicity Testing
Reliable biological evaluation begins with understanding how materials interact with living cells at the most fundamental level. Cytotoxicity Testing assesses whether a material, extract, or chemical leachable induces cell death, inhibits proliferation, disrupts metabolic activity, or causes adverse morphological changes in cultured cells.
At Materials Metric, cytotoxicity testing is performed in accordance with ISO 10993-5 and FDA guidance, providing early, sensitive detection of potential biological hazards arising from residual solvents, unreacted monomers, processing additives, surface contamination, or degradation by-products. These assays deliver rapid, quantitative insight into cellular response, enabling informed decision-making during material selection, formulation development, risk assessment, and regulatory submission preparation before advancing to more complex in-vitro or in-vivo studies.
Uses of Cytotoxicity Testing
• Early screening of new materials and formulations
• Assessment of surface treatments, coatings, and residual processing agents
• Evaluation of extractable chemicals following ISO 10993-12 extraction conditions
• Batch-to-batch biological safety verification
• Supporting FDA submissions and ISO 10993 biological evaluation plans
• Identifying root causes when other biocompatibility endpoints fail

Applications
• Medical device polymers, elastomers, adhesives, coatings
• Hydrogels, composites, 3D-printed materials
• Dental materials and implant surfaces
• Drug-device combination products
• Packaging, tubing, and contact materials
• Regenerative medicine and biomaterial R&D
Sample Analysis Process
1. Study Planning
- Selection of assay format (direct contact, indirect, or extract-based)
- Cell line selection (commonly L929 fibroblasts)
- Determination of extraction conditions per ISO 10993-12
2. Sample Preparation
- Preparation of materials or extracts under controlled conditions
- Sterilization or cleaning (as required)
- Setup of direct or indirect contact configurations
3. Exposure & Measurement
- Incubation of cells with material/extract
- Quantification via metabolic assays (MTT, XTT, AlamarBlue, LDH)
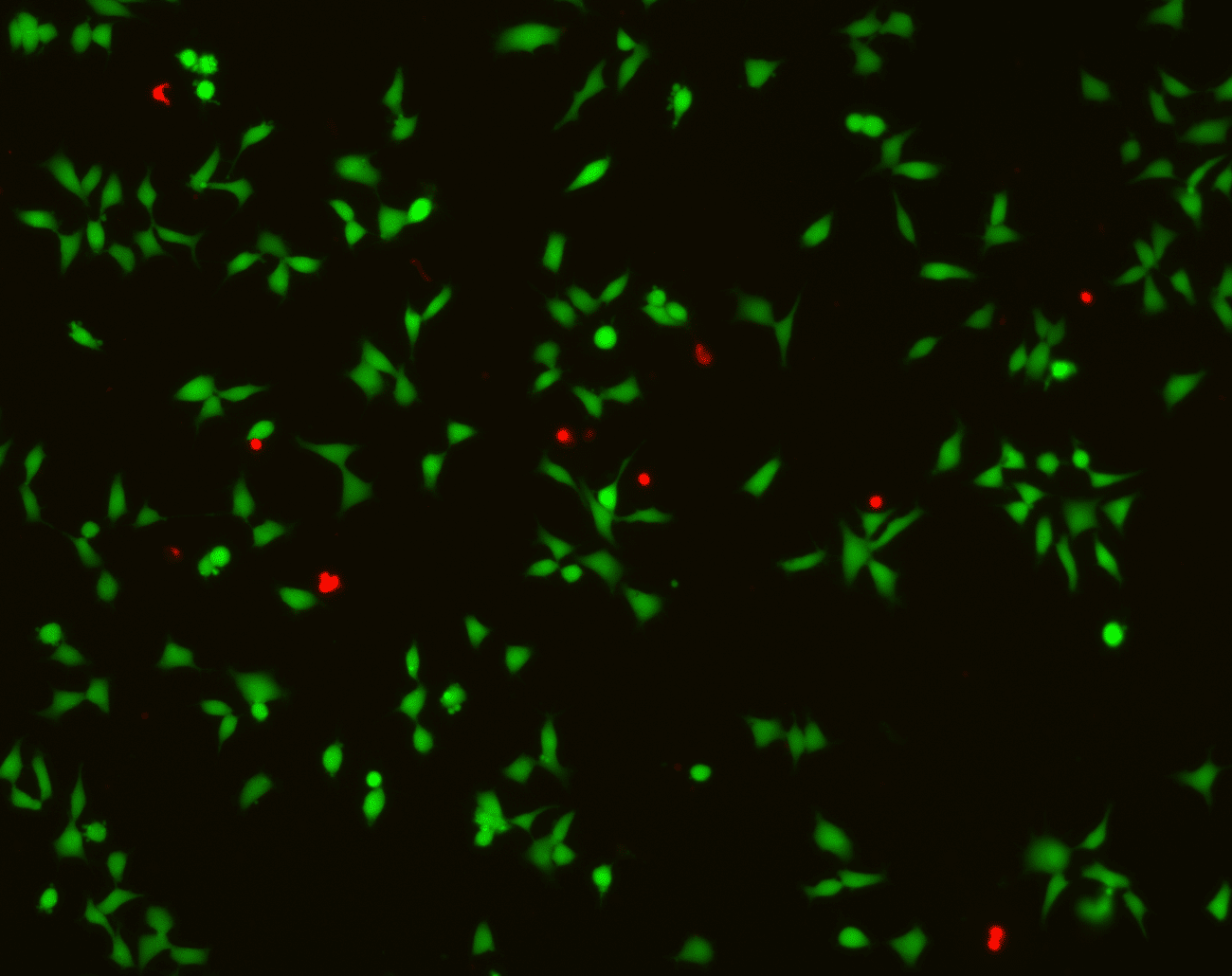
- Microscopic evaluation of cell morphology
- Scoring of cytotoxic response based on viability reduction
4. Data Analysis & Reporting
- Quantitative viability (%)
- Cytotoxicity grade and classification
- Annotated microscopy images
- Interpretation relative to ISO 10993-5 acceptability criteria
- Recommendations if cytotoxic response is observed

Why Choose Materials Metric for Cytotoxicity Testing
Materials Metric delivers robust, regulator-ready cytotoxicity testing with deep scientific interpretation and strict quality oversight.
We provide:
• ISO 10993-5 Compliant Cytotoxicity Workflows
Our methods follow global standards to ensure your results are accepted by FDA, EU MDR, and international regulatory bodies.
• ISO 9001:2015 Accredited Quality System
Every step from extraction to reporting is controlled, traceable, and audit-ready, supporting smooth regulatory review.
• High-Sensitivity Quantitative Assays
We use validated metabolic and morphology-based assays to detect subtle cytotoxic effects that routine screens may miss.
• Expert Interpretation of Complex Results
Our biomaterials and toxicology specialists provide clear explanations of borderline findings, failure patterns, and root-cause hypotheses.
• Integrated Chemical + Biological Correlation
Cytotoxicity outcomes are often driven by chemistry.
We can correlate your results with:
• extractables & leachables
• surface chemistry
• thermal & mechanical behavior
• material processing history
• Strategic Support for FDA & ISO Submissions
We help you navigate requirements, prepare Biological Risk Assessments, and design next-step testing if needed.
Materials Metric ensures your cytotoxicity evaluation is accurate, defensible, and aligned with your regulatory and R&D goals.
