Chemical analysis is the process of determining what substances make up a material and how much of each component is present. You can think of it as detective work at the molecular level. Scientists use specialized instruments and procedures to identify unknown compounds, measure element concentrations, verify material purity, and detect contaminants that could compromise product quality or safety. This work underpins everything from pharmaceutical development to aerospace manufacturing.
This guide walks you through the fundamentals of chemical analysis. You’ll learn why it matters for quality control and innovation, discover the difference between qualitative and quantitative approaches, explore the most common analytical techniques, and see how industries apply these methods to solve real problems. Whether you need to characterize a new material, troubleshoot a manufacturing issue, or meet regulatory requirements, understanding these core concepts will help you make better decisions about testing strategies and data interpretation.
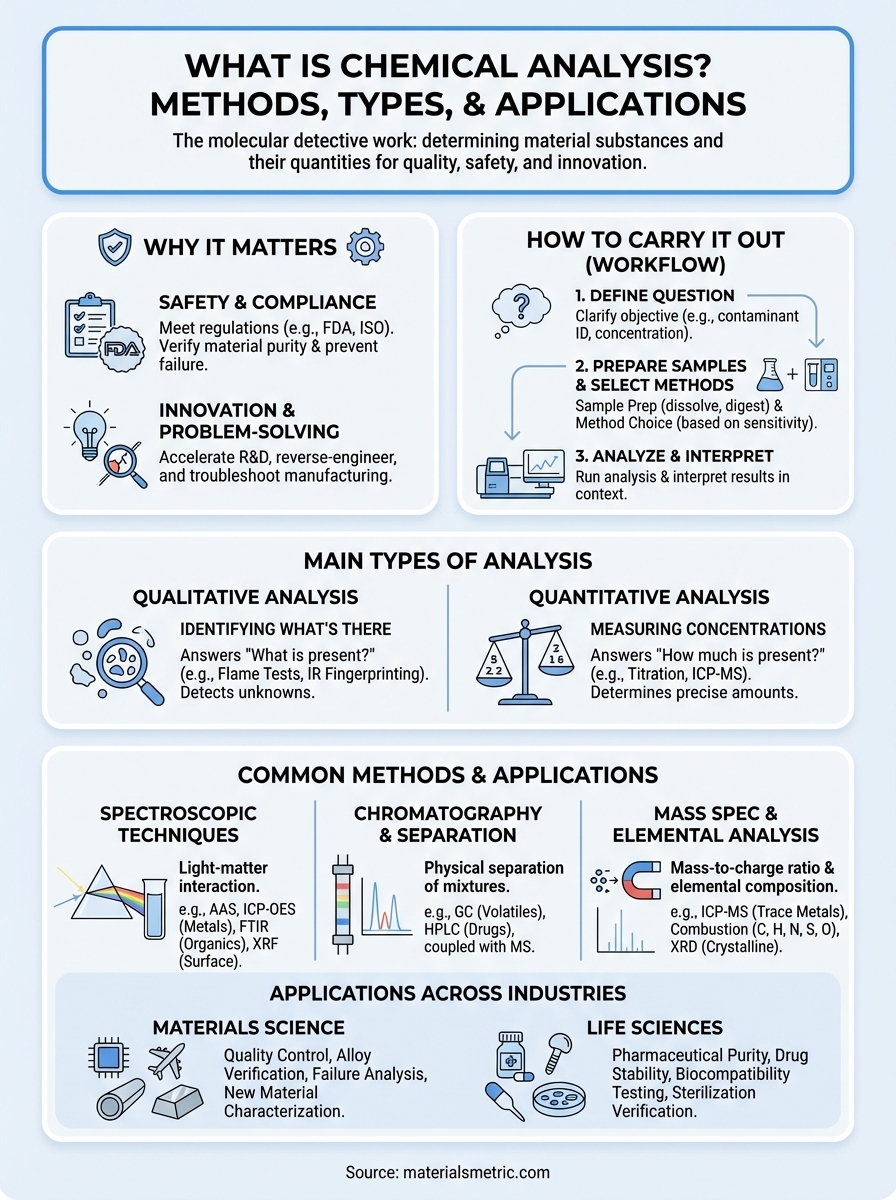
Why chemical analysis matters
Chemical analysis gives you the data you need to make confident decisions about materials, products, and processes. When you understand exactly what a substance contains and in what quantities, you can predict how it will behave in real-world conditions. This knowledge prevents costly failures, protects end users from harm, and accelerates your path from concept to market. Without accurate chemical data, you’re essentially guessing about material performance, safety profiles, and regulatory compliance.

Protecting safety and meeting regulations
Regulatory agencies demand proof that your products meet strict chemical composition standards before you can sell them. A medical device manufacturer must demonstrate that materials won’t leach harmful substances into the body. An aerospace supplier needs to verify that alloy compositions match specifications down to trace elements. Chemical analysis provides the documented evidence that satisfies FDA requirements, ISO standards, and industry-specific regulations. One contamination event or compositional deviation can trigger recalls, legal liability, and reputation damage that far exceeds the cost of thorough testing.
Regular chemical testing catches problems before they reach customers or regulators.
Accelerating innovation and solving problems
When you develop new materials or optimize existing formulations, chemical analysis reveals how compositional changes affect performance characteristics. You can identify unknown contaminants causing production defects, reverse-engineer competitor materials, or verify that manufacturing processes maintain consistency batch after batch. This diagnostic capability shortens R&D cycles, reduces waste from failed experiments, and gives you the technical foundation to patent innovations or defend intellectual property claims.
How to carry out chemical analysis
Carrying out chemical analysis follows a structured workflow that transforms raw samples into reliable data. You begin by defining your analytical objective, then select appropriate methods, prepare samples carefully, run the analysis, and interpret results within the context of your original question. Each step demands attention to detail because errors compound quickly. A poorly prepared sample or miscalibrated instrument can invalidate an entire test run and waste both time and resources.
Define your analytical question
Before you touch any equipment, you need to clarify exactly what information you’re seeking. Are you identifying an unknown contaminant that’s causing production failures? Measuring the concentration of a specific element to verify batch consistency? Determining the complete elemental composition of a new alloy? Your analytical question determines which techniques you’ll use and how you’ll interpret the data. A vague question like "tell me about this material" leads to unfocused testing that generates mountains of irrelevant data while missing critical details.
The sharper your analytical question, the more efficiently you reach actionable answers.
Prepare samples and select methods
Sample preparation often makes the difference between accurate results and misleading data. You may need to dissolve solid materials in appropriate solvents, digest samples with acids, or section them for microscopy. The goal is to present your material to the analytical instrument in a form that produces reliable measurements while preserving the chemical information you need. Your choice of analytical method depends on your question, sample type, required sensitivity, and available equipment. Understanding what is chemical analysis in your specific context helps you match the right technique to your problem. Some questions need one targeted test, while complex materials require multiple complementary methods to build a complete picture. You also consider factors like detection limits, matrix effects, and whether you need to preserve the sample for additional testing.

Main types of chemical analysis
Chemical analysis splits into two fundamental categories that answer different questions about your materials. Qualitative analysis tells you what substances are present, while quantitative analysis measures how much of each component you have. You typically run qualitative tests first to identify unknown materials, then follow up with quantitative methods to determine precise concentrations. Understanding what is chemical analysis in both forms gives you the complete picture you need for quality control, materials characterization, and troubleshooting. Most real-world projects require both approaches working together to produce actionable results.
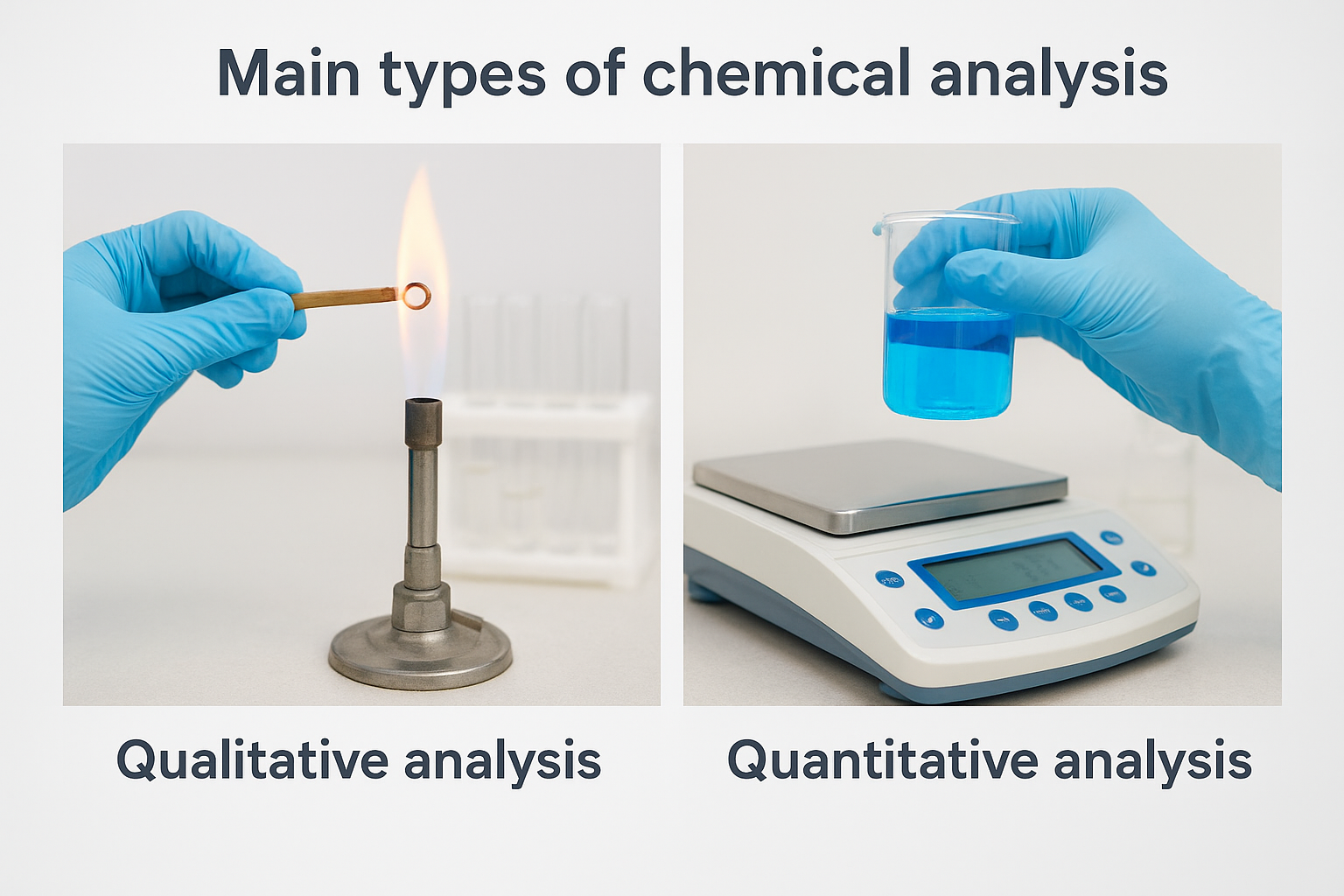
Qualitative analysis: identifying what’s there
Qualitative methods detect and identify the chemical components in your sample without measuring exact amounts. You might use these techniques when you find unexpected contamination on a production line, need to verify that raw materials match supplier specifications, or want to reverse-engineer a competitor’s formulation. Common qualitative approaches include flame tests that reveal metal ions through characteristic colors, spectroscopic techniques that produce unique chemical fingerprints, and separation methods that isolate individual compounds for identification. These tests answer yes-or-no questions: Does this coating contain lead? Is this polymer contaminated with silicone? Did the sterilization process alter the material chemistry?
Qualitative analysis gives you the starting point for every deeper investigation.
Quantitative analysis: measuring concentrations
Quantitative methods determine the precise amount of each identified substance in your material. You need these measurements to verify that active ingredients meet dosage specifications, ensure that trace contaminants stay below regulatory limits, or track how processing conditions affect composition. Techniques like titration, gravimetric analysis, and instrumental methods produce numerical data with known precision and detection limits. A pharmaceutical manufacturer uses quantitative analysis to confirm that each batch contains exactly 500 mg of active drug per tablet. An aerospace supplier measures titanium alloy composition to within 0.01% to meet strict performance requirements. Your quantitative results become the documented proof that products meet specifications and regulations.
Common methods and techniques
Modern laboratories rely on a core set of analytical techniques that cover most materials characterization needs. Each method exploits different physical or chemical properties to extract information from your samples. You’ll encounter instruments based on light interaction, mass measurement, separation principles, and thermal behavior. Understanding what is chemical analysis means recognizing that no single technique answers every question. Complex materials often require multiple complementary methods to reveal their complete composition, structure, and properties. Your choice of technique depends on the elements or compounds you’re targeting, the concentration ranges you need to measure, and whether you can destroy the sample during testing.
Spectroscopic techniques
Spectroscopy measures how materials interact with electromagnetic radiation across different wavelengths. Atomic absorption spectroscopy (AAS) and inductively coupled plasma optical emission spectrometry (ICP-OES) excel at detecting and quantifying metals in solutions. You dissolve your sample, introduce it into a high-energy plasma or flame, and measure the characteristic light that atoms emit or absorb. These methods routinely measure concentrations down to parts per million or lower. Infrared spectroscopy (IR and FTIR) identifies organic functional groups by measuring molecular vibrations, making it invaluable for polymer characterization and contamination identification. X-ray fluorescence (XRF) provides rapid, non-destructive elemental analysis directly on solid surfaces, perfect for screening incoming materials or checking coating thicknesses.

Spectroscopic methods give you rapid answers about both elemental and molecular composition.
Chromatography and separation methods
Chromatography physically separates complex mixtures into individual components before detection. Gas chromatography (GC) and liquid chromatography (LC) push your sample through columns packed with materials that interact differently with each compound. Components that interact strongly move slowly, while weakly interacting substances pass through quickly. This separation lets you identify and quantify dozens or hundreds of compounds in a single run. Pharmaceutical companies use high-performance liquid chromatography (HPLC) to verify drug purity and measure degradation products. Environmental labs employ GC to detect volatile organic contaminants at trace levels. When you couple chromatography with mass spectrometry detection, you gain both separation power and molecular identification in one workflow.
Mass spectrometry and elemental analysis
Mass spectrometry (MS) measures the mass-to-charge ratio of ions to identify molecules and determine their structure. Inductively coupled plasma mass spectrometry (ICP-MS) achieves detection limits in the parts per billion range for most elements, making it the go-to method for trace metal analysis. You use combustion analyzers to measure carbon, sulfur, nitrogen, oxygen, and hydrogen in metals and organic materials by burning samples at high temperatures and detecting the resulting gases. X-ray diffraction (XRD) identifies crystalline phases in materials by measuring how X-rays scatter off ordered atomic planes, essential for characterizing ceramics, minerals, and metal alloys.
Applications in materials and life sciences
Chemical analysis drives critical decisions across diverse industries where material performance and biological safety directly impact product success and human health. When you work with advanced materials, medical devices, or pharmaceutical products, you rely on analytical data to verify compositions, detect impurities, demonstrate regulatory compliance, and solve manufacturing problems. Understanding what is chemical analysis means recognizing its role as the foundation for quality assurance, innovation, and risk management. These techniques transform abstract chemical properties into concrete measurements that guide engineering choices, clinical studies, and regulatory submissions.
Materials characterization and quality control
You use chemical analysis to verify that metals, polymers, ceramics, and composite materials meet specification requirements throughout the manufacturing process. An aerospace manufacturer analyzes titanium alloy compositions to ensure that every batch delivers the strength and corrosion resistance that flight-critical components demand. Semiconductor fabricators test silicon wafer purity because trace contaminants at parts-per-billion levels cause device failures. When production defects appear, chemical analysis identifies the root cause by detecting unexpected elements, measuring composition drift, or revealing surface contamination. Materials scientists characterize new formulations by mapping elemental distributions, measuring crystalline phases, and tracking how processing conditions alter chemistry. This data feeds directly into product development cycles, helping you optimize properties while maintaining manufacturability.
Chemical testing transforms material unknowns into engineering certainties.
Biomedical and pharmaceutical applications
Pharmaceutical companies depend on chemical analysis to demonstrate that drug formulations contain precise amounts of active ingredients, remain stable over shelf life, and carry no harmful impurities. You test medical devices and implants for biocompatibility by measuring what substances leach into biological fluids, ensuring that materials won’t trigger adverse reactions when placed in the human body. Chemical characterization verifies that sterilization processes haven’t degraded polymers or altered surface chemistry. Tissue engineers analyze scaffold materials to confirm that compositions support cell growth and tissue integration. Antimicrobial coatings undergo rigorous testing to quantify active agent concentrations and prove long-term effectiveness. Regulatory pathways like FDA approval require extensive chemical data documenting material safety, consistency, and performance under physiological conditions.
Bringing it all together
Understanding what is chemical analysis gives you the foundation to make informed decisions about testing strategies and data interpretation across your materials projects. You now know the difference between qualitative and quantitative approaches, recognize the most common analytical techniques, and see how industries apply these methods to verify quality, meet regulations, and drive innovation. Every successful material characterization project starts with clear analytical questions that guide method selection, sample preparation, and result interpretation to deliver actionable answers.
Your next step depends on your specific challenge. If you need to characterize new materials, troubleshoot manufacturing issues, or generate regulatory compliance data, Materials Metric provides the analytical expertise and advanced instrumentation to deliver accurate, defensible results. Our multidisciplinary team translates complex chemical data into actionable insights that accelerate your development timelines and support critical commercial milestones.